

BIs are made using bacterial spores that are even harder to kill than any potential remaining microorganisms that may have survived the surgical equipment decontamination process. If the retest confirms a positive spore growth, then the sterilizer must remain out of service and all instruments that were sterilized by that machine must be recalled from use and re-sterilized through a different sterilizer to confirm that they are safe for patient care. All steam, ETO, and other low-temperature sterilizers are tested with biological and chemical indicators upon installation, when the sterilizer is relocated, redesigned, after major repair and after a sterilization failure has occurred to ensure they are functioning prior to placing them into routine use. A biological indicator (BI) is the only way to directly measure the lethality of a sterilization cycle. Remember to document both the failed test and the passing retest in this scenario. Most failed spore tests are due to operator error, so a passed retest confirms that the sterilizer is safe to use.
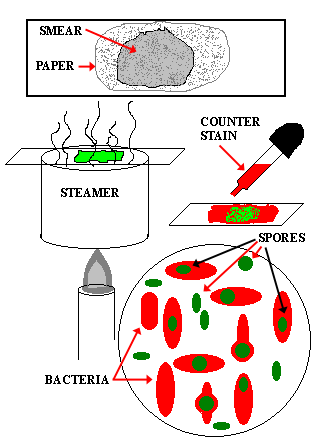
The sterilizer must be removed from service until you get a passing result from the retest. If a positive test is reported on a sterilizer, immediately retest the sterilizer using the same cycle that produced the positive result.

that a test cycle of an autoclave was run with a biological indicator (spore. Remove any sterilizer with a positive test A vendor who processes biological indicators usually provides the needed.


 0 kommentar(er)
0 kommentar(er)
